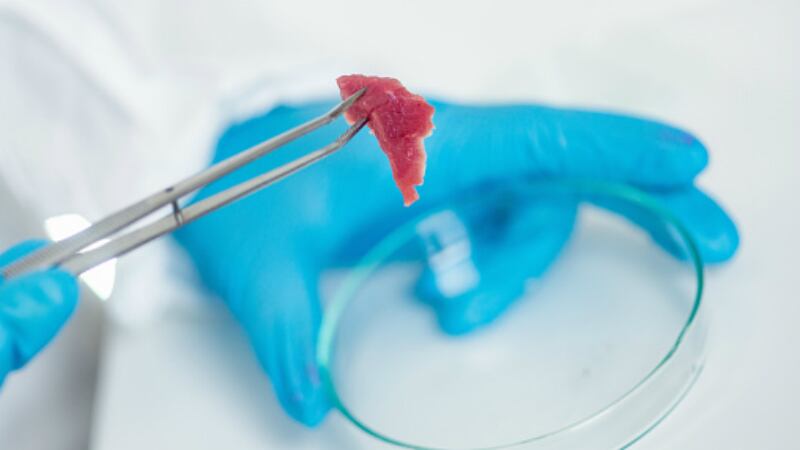
This initiative falls under the purview of the newly-established Singapore Food Agency (SFA), and public consultations on this topic have been ongoing since 2018, when all food-related functions were still governed by the then-Agri-Food & Veterinary Authority of Singapore (AVA).
In a statement to FoodNavigator-Asia, SFA has revealed that safety assessments are currently a big part of the agency’s focus in determining how these new products should be regulated.
“Production of novel foods (e.g. cell-based meat, alternative proteins) for human consumption is a nascent industry,” said SFA.
“As novel foods do not have a history of being consumed by humans as food, they may pose food safety risks and their safety needs to be assessed before they can be allowed to be used in food for sale in Singapore.”
The agency added that in such safety assessments, items of the most interest for scrutiny for these novel foods include materials that go into the process of manufacturing (such as the type of cells, growth media and scaffolding materials for cell-based meat) and how manufacturing processes are controlled to prevent food safety risks.
“Food safety should be an integral element as companies develop food products,” SFA added.
Evaluations have taken place regarding the safety and sales of ‘innovative food products’ in Singapore since last year, including JUST Egg and the Impossible Burger, in addition to the aforementioned public consultations.
No results from these have been released as of yet, nor has a specific timeline been revealed. but SFA will be rolling out a novel foods regulatory framework which would require companies to seek pre-market approval and go through a safety assessment for these foods that do not have a history of use as food.
“Novel food products will be assessed on a case-by-case basis before import or sale is allowed,” the agency added.
“Companies that wish to import or manufacture novel food products for sale are required to seek SFA’s approval and provide the necessary supportive evidence of food safety for our assessment.”
More details on requirements
According to official documentation on the novel food safety assessment process, which FoodNavigator-Asia has viewed courtesy of SFA, the assessment covers food safety issues which includes potential toxicity, allergenicity, safety of its production method, and dietary exposure.
“SFA considers novel foods to be foods and food ingredients that do not have a history of safe use, [which means these] have been consumed as an ongoing part of the diet by a significant human population, for a period of at least 20 years and without reported adverse human health effects,” said SFA via the document.
“Novel foods may also include compounds that are chemically identical to naturally-occurring substances, but produced through advances in technology.”
In a specific section dedicated to cell-based clean meat, very detailed information on the overall manufacturing process, materials and product is required, including cell lines, medium, scaffolding, possible hazards, allergen profiling, product nutritional composition and more.
At the moment, SFA will not be charging any fees for the evaluation of applications for the use of novel foods, but it does estimate a timeline of between three to six months to complete any novel food evaluation.
“In order to avoid delays, [food innovators] are encouraged to consult SFA early in their product development process to understand the information that would be required to be submitted in order to substantiate the safety of their novel food,” said the agency.
“As novel food is a rapidly evolving area, SFA will periodically update and revise this document to facilitate the safety assessments by the industry and ensure food safety.”
A novel food safety expert working group is also being established to provide advice.
More details can be found in the SFA guidance document here.